Shattering centuries-old sterilization with
FemBloc
Safe & Effective
The next advancement of non-surgical permanent birth control is here.
Clinical Data
The next advancement of non-surgical permanent birth control is here.
Read Clinical Data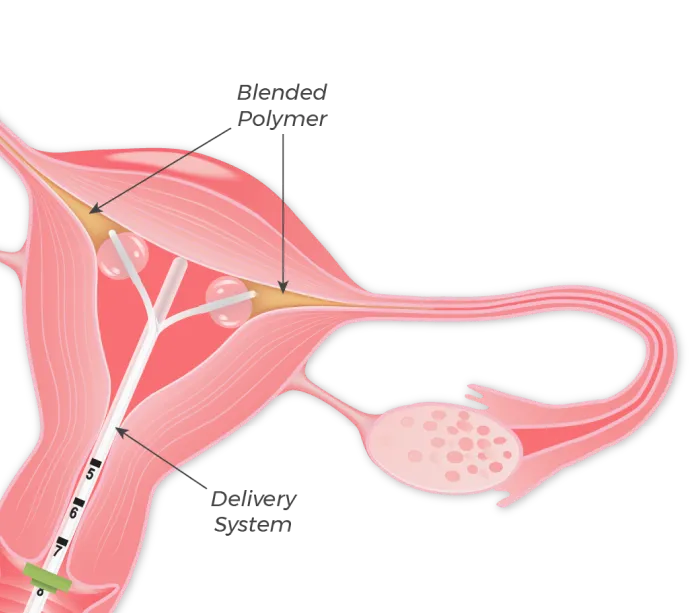
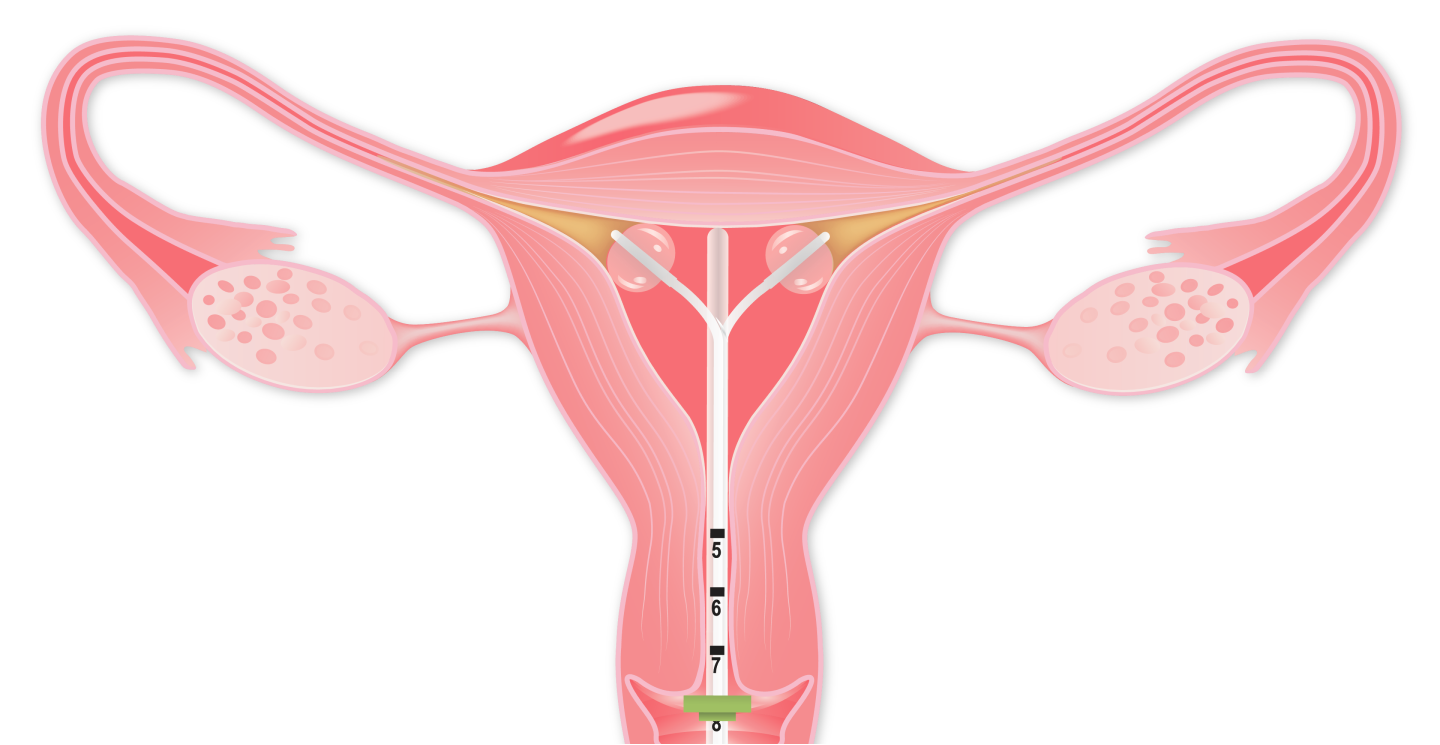
FemBloc involves the delivery of our proprietary blended polymer precisely to both fallopian tubes simultaneously by transcervical placement of our delivery system, consisting of two balloon catheters positioned in each cornu.
FemBloc allows for expansion of practice services with an affordable, accessible, non-surgical permanent contraceptive solution with demonstrated safety and effectiveness.
(for subjects who met trial eligibility and were determined bilaterally blocked after confirmation test)
FemBloc demonstrated a significantly lower pregnancy rate than the historical control.
Based on real-world effectiveness of surgical (laparoscopic) sterilization evaluated in a recent large study (N=23,965), which concluded:
Note: CREST study (U.S. Collaborative Review of Sterilization) was conducted between 1978 and 1981 (N=10,685) and is considered outdated with 28% of the sterilization techniques examined no longer used.2
There were no reports of serious adverse events (n=0/229)1
Participants are currently being enrolled in the FINALE pivotal clinical trial (NCT05977751) for U.S. approval.
1Liu, J. H., Blumenthal, P. D., Castano, P. M., Chudnoff, S. C., Gawron, L. M., Johnstone, E. B., Lee-Sepsick, K. (2025). FemBloc Non-Surgical Permanent Contraception for Occlusion of the Fallopian Tubes. J Gynecol Reprod Med, 9(1), 01-12. doi: 10.33140/JGRM.09.01.05.
2Gariepy AM, Lewis C, Zuckerman D, et al. Comparative Effectiveness of Hysteroscopic and Laparoscopic Sterilization for Women: A Retrospective Cohort Study. Fertility and Sterility, 2022, 117(6):1322-1331. doi: 10.1016/j.fertnstert.2022.03.001.
Only a fraction undergo surgical sterilization for various reasons, including surgical risks.
Consider Non-Surgical Permanent Birth Control for:
Learn how to perform the FemBloc procedure at your convenience. Our FemBloc online training platform details the procedure step-by-step and provides valuable resources.